Investigation of causes of male azoospermia and oligospermia
Investigation of causes of male azoospermia and oligospermia
According to the survey data of the World Health Organization, about 15% of couples of childbearing age have fertility disorders, of which about 50% are caused by male factors. Among the known genetic factors that cause male infertility, the two most common are Y chromosome Microdeletion and Klinefelter's syndrome (XXY).
Y chromosome microdeletion accounts for about 10-15% of patients with azoospermia or oligospermia, and has become a routine inspection item for male infertility patients.
Kit can detect complete deletion of each AZF region, partial deletion and duplication of AZFb/c region, and abnormal number of sex chromosomes (such as Klinefelter syndrome)
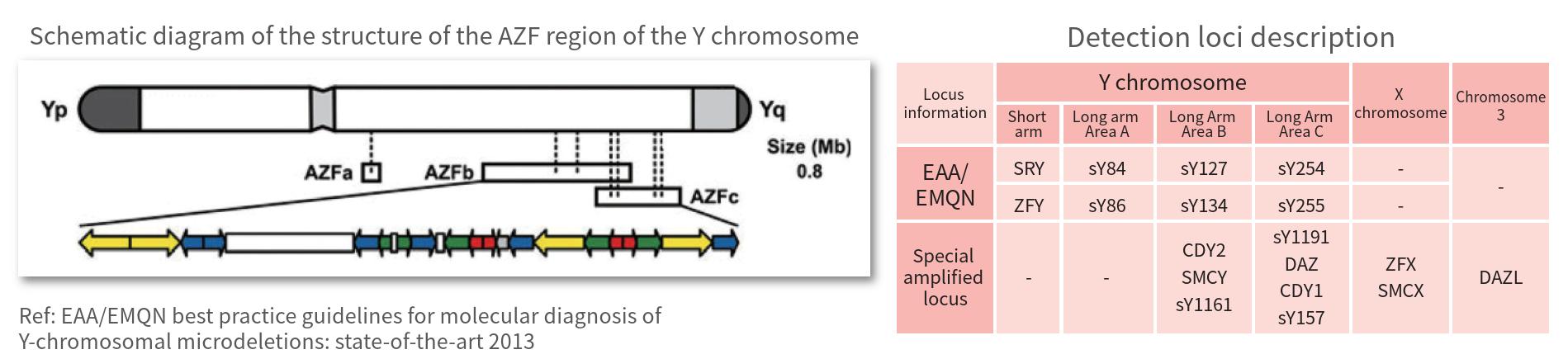
Detection of loci In addition to complying with the EAA/EMQN recommended system, it also expands the AZFb/c region and the X chromosome, a total of 16 gentic loci can be detected
Single-tube amplification, which is simple and fast to operate
Professional software assists result analysis and interpretation, which is fast and easy
The Y chromosome has a large number of repetitive gene sequences and palindromic structures. The three regions with the highest deletion rate affecting spermatogenesis are named as AZFa, AZFb and AZFc. The deletion of any one of them may lead to decreased fertility or infertility. Accordingly, in 2004, the European Association of Andrology and the European Molecular Genetics Laboratory Quality Control Network (EAA/EMQN) published the "Guidelines for the Molecular Diagnosis of Y Chromosome Microdeletions", suggesting that a total of 6 STS sites in each AZF region be detected Y chromosome microdeletion. This kit also adds sites that can be used to detect partial deletions, duplications, and abnormalities in the number of sex chromosomes (such as Klinefelter's syndrome) in the AZFb/c region.


PCR instrument: Life Technologies Holdings Pte Ltd: 9700, Veriti DX;
Genetic analyzer: Life Technologies Holdings Pte Ltd: 3730xL Dx, 3500 Dx; Seqstudio
Fertil Steril. 2002 May;77(5):873-82.
Fertil Steril. 2010 Jan;93(1):1-12.
Andrology. 2014 Jan;2(1):5-19.
※ This product is only for scientific research use, and this information is only for reference by relevant medical professionals. Please refer to the instruction manual for details of contraindications or precautions.
[ENG]Y_chromosome_microdeletion.pdf:Reference:[ENG]Y_chromosome_microdeletion.pdf