■ Prenatal screening, prenatal diagnosis, carrier screening
■ Investigation of causes of birth defects
■ Prenatal screening, prenatal diagnosis, carrier screening
■ Investigation of causes of birth defects
Abnormal chromosome number is one of the important causes of birth defects. Trisomy 21, trisomy 18, trisomy 13 and X/Y chromosomal abnormalities account for 95% of neonatal chromosomal abnormalities.
There are many diagnostic methods for chromosomal aneuploidy. Multiplex fluorescent PCR & capillary electrophoresis is a diagnostic technique due to its advantages of accuracy, rapidity, and low price. [1-2] In 2004, the National Screening Committee of the United Kingdom suggested that the screening of high-risk pregnant women with Down syndrome should not use the method of karyotype analysis, but directly confirm whether the fetus has Down syndrome through multiple fluorescent PCR & capillary electrophoresis.
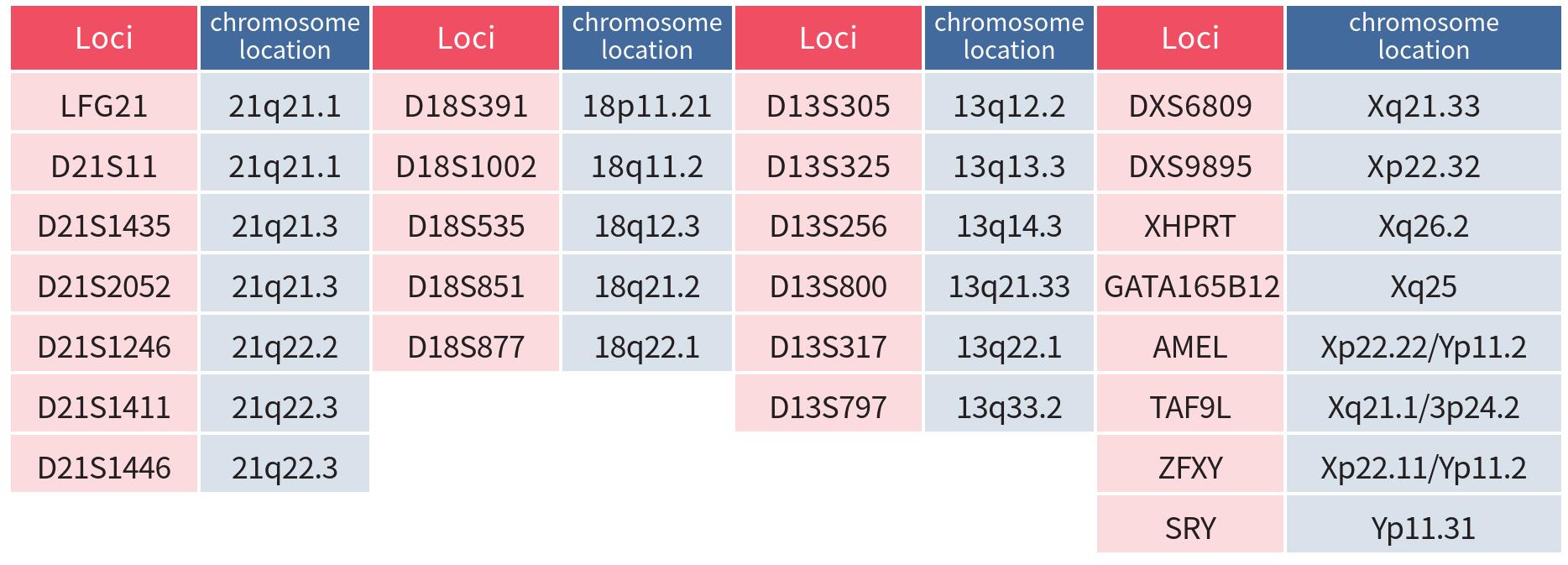
This kit can detect 26 genetic loci for abnormal number of chromosomes of 21, 18, 13, X and Y, ≥ 4 STR loci tested per chromosome.
Single-tube amplification, takes only 4 hours, suitable for automatic and batch detection.
Template DNA amount as low as 1ng gDNA.
Professional software assists in analysis and interpretation of results, fast and accurate.
Effectively avoids PCR product contamination.
After DNA in the sample was extracted, and multiplex fluorescent PCR was used to detect 7 STR loci on chromosome 21, 5 STR loci on chromosome 18, 6 STR loci on chromosome 13, 4 STR loci on chromosome X and 4 STR loci on chromosome 13. Gender-related STS loci were amplified, and the amplified products were detected by capillary electrophoresis using a genetic analyzer, and the results were analyzed using professional software.


PCR machine: Life Technologies Holdings Pte Ltd: Veriti, Veriti Dx, 9700
Genetic analyzer: Life Technologies Holdings Pte Ltd: 3500 Dx, 3500 xL Dx; Sequstudio
[1] Zhu Yuning. Research on Fetal Chromosomal Abnormalities and Appropriate Prenatal Diagnosis Techniques[D]. Zhejiang University, 2015.
[2] Zhuan Jia. Rapid diagnosis of fetal chromosomal aneuploidy using QF-PCR technology[D]. Hebei Medical University, 2012.
※This product is only for scientific research use, and this information is only for reference by relevant medical professionals. Please refer to the instruction manual for details of contraindications or precautions.
[ENG]Aneuploid.pdf:Reference:[ENG]Aneuploid.pdf