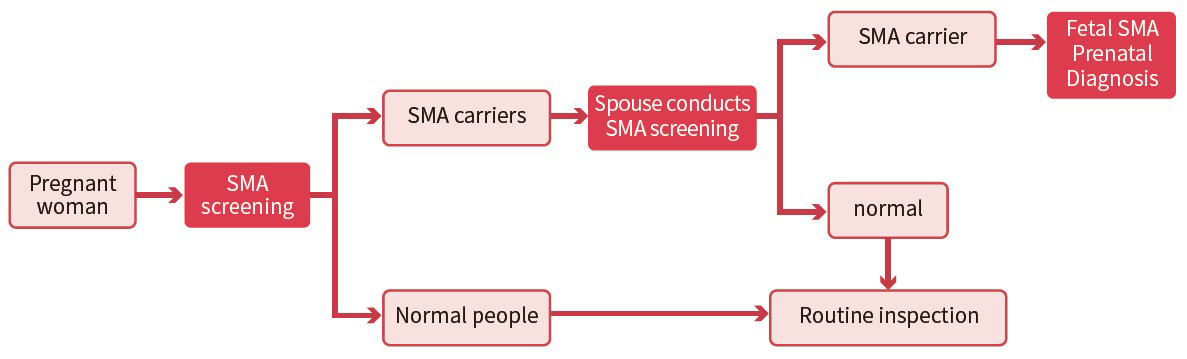
Spinal muscular atrophy (SMA) is the most common fatal autosomal recessive genetic disease in infants and young children, with a carrier rate of about 1/50 in the population. The clinical manifestations are progressive, symmetrical, proximal limb muscle weakness and muscle atrophy, and are divided into types 0-IV according to the time of onset and clinical manifestations. About 95% of SMA patients are caused by homozygous deletion of exon 7 of SMN1, and the copy number of SMN2 affects the prognosis of the disease.
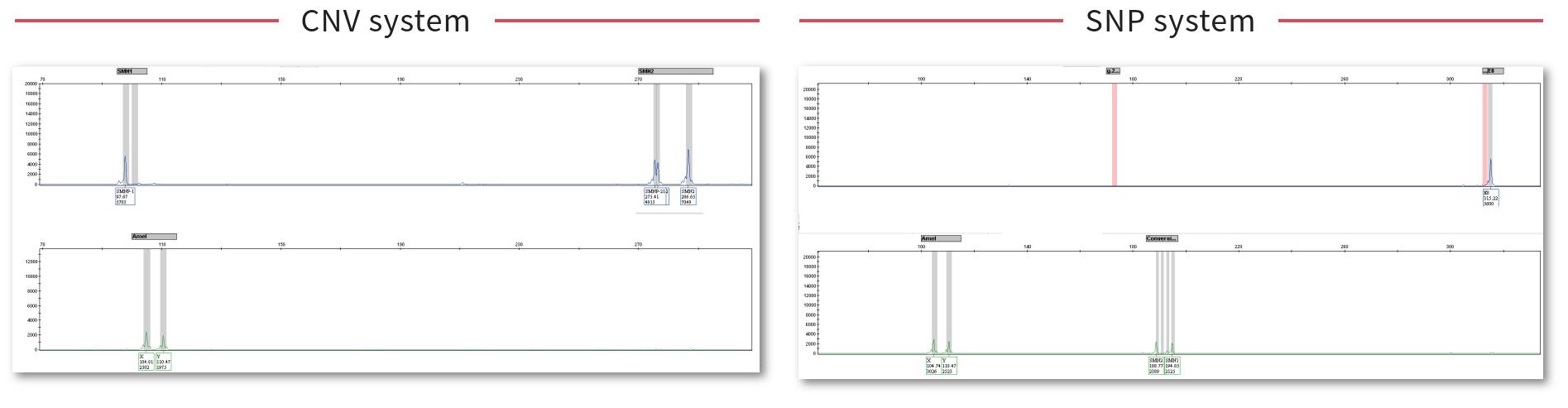
The kit uses fluorescent PCR-capillary electrophoresis to detect the copy numbers of SMN1 and SMN2 and the SNP loci related to "2+0" carriers, which can accurately distinguish healthy people, carriers and patients, and is used for SMA carriers Screening and auxiliary diagnosis of patients.
The kit is stable and can accurately distinguish healthy people, carriers, and patients
Can detect SMN1, SMN2 copy number and "2+0" carrier-related point mutations
Based on fluorescent PCR-capillary electrophoresis, the whole detection process takes about 4 hours


PCR instrument: Life Technologies Holdings Pte Ltd: 9700, Veriti DX;
Genetic analyzer: Life Technologies Holdings Pte Ltd: 3730xL Dx, 3500 Dx; Seqstudio.
24 reactions / kit, 48 reactions / kit
※This product is only for scientific research use, and this information is only for reference by relevant medical professionals. Please refer to the instruction manual for details of contraindications or precautions.
[ENG]SMA.pdf:Reference:[ENG]SMA.pdf