statin and lbgl medication guidance
statin and lbgl medication guidance
Warfarin is a first-line drug for the prevention and treatment of thromboembolic diseases, but its drug reactions vary greatly. The difference in the stable dose of warfarin between different individuals can be more than 20 times, and the treatment window is narrow, so the dose is difficult to grasp, especially in the early stage of warfarin anticoagulation treatment, which can easily lead to serious bleeding complications. A large number of studies have shown that the pharmacokinetic process of warfarin is closely related to the genotyping of CYP2C9 and VKORC1. In 2010, the FDA added the starting dose selection table to the warfarin manual, and recommended CYP2C9 and VKORC1 genetic testing before medication.
Clinical studies show that compared with parallel control groups that do not consider individual gene polymorphisms, genetic algorithms that consider CYP2C9 and VCORK1 gene polymorphisms are closer to the actual dose required by patients: the time ratio of INR value out of range is reduced (42% to 31% at 1 month and 4 at 3 months. 2% to 30%), the time-to-time ratio of effective drug concentration increased (58% to 69% at 1 month, 59% to 71% at 3 months).
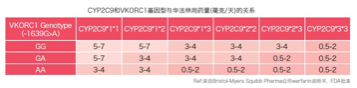
As shown in the figure below, the genetic algorithm is adjusted for people of
different genotypes on the basis of clinical algorithms to make it suitable for the drug metabolism needs
of different groups of people.


Clopido is a widely used antiplatelet agglutination drug for the treatment of acute coronary syndrome, atherosclerosis and other cardiovascular diseases. Clopido is a progen drug that can only be effective after being metabolized into an active product by liver cytochrome P450 enzyme (mainly CYP2C19 enzyme). Different individuals have different reactions to clopido. About 40% of Asian patients produce clopido resistance, mainly due to weak metabolism caused by mutations in the CYP2C19 gene. CYP2C19 has at least 36 alleles, of which CYP2C19*2 and CYP2C19*3 account for more than 99% of the weak metabolic phenotypes in the Asian population. CYP2C19*17 is the genotype with ultra-fast activity found so far. In 2010, the FDA issued a black-frame warning, clearly recommending that doctors conduct CYP2C19 genotyping tests for patients who will take clopido, and implement personalized drugs for patient genotypes.
Clinical studies have shown that in weak metabolic individuals caused by CYP2C19 gene mutations, the risk of embolism re-formation increases, the risk of cardiovascular and cerebrovascular events increases, and the case fatality rate increases. For such individuals, the dose of clopido (load dose is 300mg or 600mg) needs to be increased to achieve the effect of blocking platelet aggregation.
The first anti-thrombotic gene detection kit based on fluorescence PCR-capillary electrophoresis in China
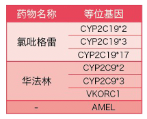
The genotype polymorphism (SNP) of 3 genes and 6 sites related to clopido and warfarin can be detected at the same time, and doctors can quickly assist doctors to customize personalized treatment plans for patients, improve the anti-embolic effect of drugs, and reduce the risk of bleeding.
※ Obtained CE certification and NMPA Class III medical device registration certificate (national equipment registration 20203400987)

No need to extract DNA, save time and effort, reduce pollution.
Combined testing of conventional hypertension drugs and folic acid related drugs, 1 tube amplification, the whole process only needs 3h.
Professional software assists in result analysis and interpretation, which is fast and easy.
2-4 ml EDTA anticoagulant blood, ≥50mg chorion sample, ≥100mg tissue sample (transported and stored at 2-4°C)
Blood stains ≥2cm2 stored in the blood card (transported and stored at room temperature)
PCR instrument and genetic analyzer (MicroreaderTM Genetic Analyzer 7010, ABI PRISM® 310, 3100 Genetic Analyzer or ABI 3130 Series, 3500 Series, 3730 Series Genetic Analyzer)
1.
Simon T, etal.
N Engl
J Med. 2009 Jan 22;360(4):363-75.
2. Hu LM, etal.
Pharmacogenomics. 2012 Nov;13(14):1571-81.
3. Sim SC,etal.
Clin Pharmacol
Ther.
2006 Jan;79(1):103-13.
4. Jessica LM, et al. N Eng
J Med. 2009;360:354-62.
5. Wei W, et al. Chin J Med Genet. 2012;29(4):420-25.
6. Rieder MJ, et al. N Engl
J Med. 2005;352:2285-93.
7. Manolopoulos
VG, et al. Pharmacogenomics. 2010;11:493-6.
8. Anderson JL, et al. Circulation. 2012 Apr 24;125(16):1997-2005.
9. Klein TE, et al. N Engl
J Med. 2009 Feb 19;360(8):753-64.
※ This information is only for the reference of relevant medical professionals. Please refer to the instructions for the use of the test kit or precautions.
※ Obtained CE certification and NMPA Class III medical device registration certificate (national equipment registration 20203400987)